FDA advisers discuss future of ‘artificial womb’ for human infants
Independent advisers to the US Food and Drug Administration will meet September 19 and 20 to discuss the regulations
By Jen Christensen, CNN
(CNN) — Independent advisers to the US Food and Drug Administration are meeting this week to discuss the regulations, ethics and possibilities of creating an artificial womb to increase the chances that extremely premature babies would survive — and without long-term health problems.
Although no such device has been tested in humans, similar ones have been used in a handful of cases to successfully develop animals. On Tuesday, during the first day of their two-day meeting, the advisers considered what human trials could look like.
An artificial womb for humans would be a scientific advance that could help solve a major health problem. Preterm births are the No. 1 killer of children under the age of 5, according to the World Health Organization.
Because a baby’s lungs and brain finish developing late in pregnancy, a child born prematurely risks a lifetime of health problems including trouble breathing, gastrointestinal issues, vision and hearing problems, developmental delays and cerebral palsy.
Prematurity has become a growing problem in the US. The number of preterm births increased from 10.1% of all babies born in 2020 to 10.5% in 2021, according to the US Centers for Disease Control and Prevention. The issue disproportionately affects African Americans, who give birth prematurely at a rate that is 50% higher than those of White and Hispanic people.
An artificial womb is not designed to replace a pregnant person; it could not be used from conception until birth. Rather, it could be used to help a small number of infants born before 28 weeks of pregnancy, which is considered extreme prematurity. Less than 1% of babies are born this early.
The earlier an infant is born, the greater the risk of death. For example, only about 30% of infants born at 22 weeks survive, and just under 56% survive birth at 23 weeks, according to a 2022 study published in the journal JAMA.
The artificial womb could be able to help the baby develop further through those vital final stages when the lung and brain are developing. Like a person’s womb, it would deliver oxygen, nutrients and hormones.
Premature babies have to stay in a neonatal intensive care unit or NICU, where they can get special nutrition, extra care for their heart, help regulating their body temperature and help with their breathing.
NICUs are regularly successful in getting premature babies through the first part of their lives, but there’s always the danger of infection at a hospital. And if the baby needs to be put on a ventilator, it may injure their tiny lungs.
Before the FDA would approve experimenting with a human in an artificial womb, scientists would have to show that the device could facilitate growth and development while reducing the rate of death and health problems, potentially compared with care with existing technology and techniques in a NICU.
The FDA’s Pediatric Advisory Committee is considering what kind of data scientists will have to show in such trials and what kind of regulations may be required, as well as what ethical considerations may need to be addressed. The committee also discussed what kind of metrics may be needed to determine the success of animal trials.
A handful of scientists have been experimenting with animals and artificial wombs. In each study, the artificial womb is constructed a little bit differently.
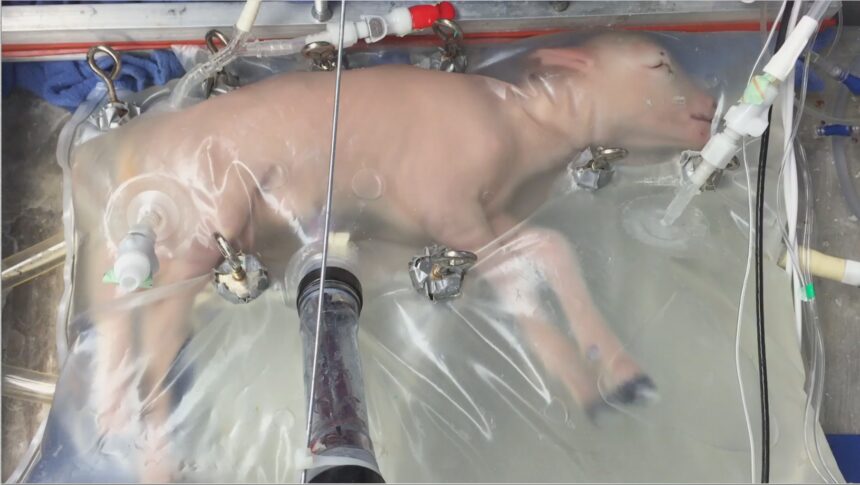
In a 2017 experiment, a group at the Children’s Hospital of Philadelphia was able to keep a developing lamb alive for 28 days in a sterilized plastic bag filled with fluid. Tubes that delivered amniotic fluid, medicine and oxygen were connected to the lamb’s umbilical cord tissue. The team saw positive growth and development in the lambs’ lungs, brains and gastrointestinal tracts.
“The technology is robust and stable,” Dr. Alan Flake, director of the Center for Fetal Research at the Children’s Hospital of Philadelphia, told the committee Tuesday. “We’ve now run over 300 lambs in the artificial womb, and the runs are generally remarkably smooth.
“We’ve observed no acute irreversible events that threatens survival,” he added. The “ultimate safety feature,” he said, is that the subject can be immediately removed and placed into standard care if necessary.
“We believe that our preclinical data supports feasibility and safety and that it’s adequate for consideration of a carefully designed clinical study of artificial womb technology,” Flake told the committee.
The group hopes to try a device they’ve been testing named the Extra-uterine Environment for Newborn Development, or EXTEND, in humans.
In a trial of what scientists at the University of Michigan call an artificial placenta, lambs survived 16 days. The team saw positive results in the development of lung function and brain development until they were able to transition to mechanical ventilation.
In a presentation to the committee Tuesday, Dr. George Mychaliska, the Robert Bartlett Collegiate Professor of Pediatric Surgery at C.S. Mott Children’s Hospital at University of Michigan Health, said the group is working on a prospective study involving this work. They foresee using this artificial placenta with humans as a kind of “rescue therapy.”
However, “we recognize there are many ethical and regulatory considerations prior to clinical translation,” Mychaliska said.
In another experiment in Japan and Australia, in an artificial womb scientists call EVE, the lamb incubated for a week and had good development in the lungs, but there was some brain injury due to a technical issue.
Scientists at the University of Toronto used fetal pigs in it experiment with an artificial placenta in a trial modeled on the lamb experiments. Pigs and humans have a similar kind of umbilical cord, but there were problems with blood circulation and some heart issues in that experiment.
Despite the setbacks, Mike Seed, head of the Division of Cardiology at the Hospital for Sick Children in Toronto, thinks they are on the right track.
“We remain extremely enthusiastic about the potential of artificial womb technology and are about to embark on a new set of experiments using a third iteration of our circuit,” he told the committee.
The FDA committee agreed that before such technology could be used with humans, scientists would have to determine the most appropriate animal model to test the artificial womb.
Experts say there may also need to be a conversation about what viability — a concept referring to the ability of a human to survive outside the womb — means.
On Tuesday, the committee discussed at length the ethics of using the technology, including what conversations doctors may have to have with parents about how successful such an intervention could be if it’s tested on humans.
The advisers also wanted to make sure that if humans were part of the trials, they would be inclusive. And they agreed that there would need to be extensive followup to determine what health effects, if any, children had in the long term.
Additionally, the committee discussed regulatory considerations and extra safeguards because trials would involve children, which by law requires extra steps to ensure safety. The advisers examined potential clinical considerations to fairly assess whether the new technology would be an advance over currently available care.
Although the two-day meeting can guide the way the FDA will move forward with regulating artificial wombs, the agency makes decisions on its own terms and does not have to follow the experts’ recommendations.
The first day of the meeting was open to the public, but the second day will be closed because the nature of the research involves proprietary information, the FDA said.
The-CNN-Wire
™ & © 2023 Cable News Network, Inc., a Warner Bros. Discovery Company. All rights reserved.