Updated Covid-19 vaccines are coming mid-September, officials say
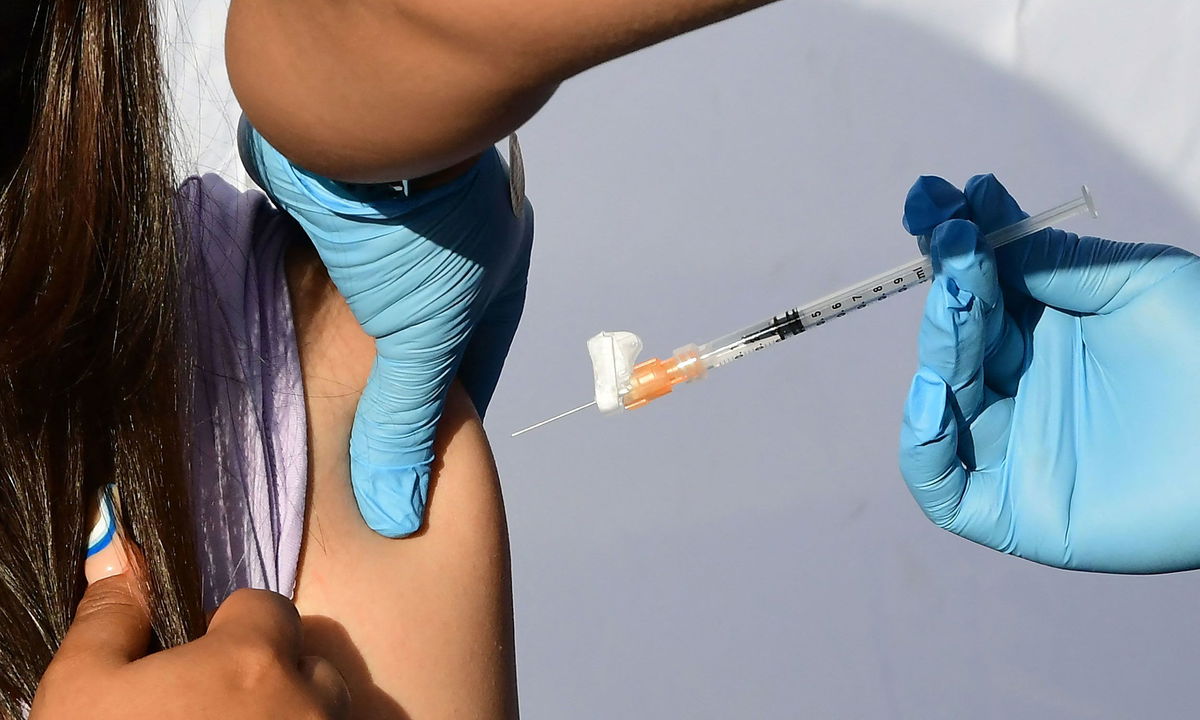
Covid-19 vaccines that have been updated to defend against XBB.1.5 are expected to be available in mid-September.
By Brenda Goodman, CNN
(CNN) — Covid-19 vaccines that have been tweaked to teach the body how to fend off the current crop of circulating variants are now expected to land in drugstores and clinics in mid-September, CDC and FDA officials said.
The officials spoke Thursday about the US government’s preparations for the fall and winter respiratory virus season on the condition that they not be named.
The US Food and Drug Administration is expected to give its nod to the updated vaccines in a few weeks. Then the Advisory Committee on Immunization Practices, a group of independent experts that advises the US Centers for Disease Control and Prevention on its vaccination decisions, will weigh the safety and effectiveness of the new shots and make recommendations for their use. After the CDC director signs off on those recommendations, the vaccines can be administered.
Officials said ACIP will meet quickly after the FDA decision in order to expedite the regulatory steps and get the vaccines to market. The advisory group is scheduled to meet to discuss Covid-19 vaccines on September 12, meaning the vaccines could become available soon after.
The announcement comes amid a late summer uptick in Covid-19. The CDC tracks the disease through hospitalizations and emergency room visits, as well as wastewater monitoring and testing of travelers at some airports. More than 12,600 Americans are hospitalized with Covid-19, and that number is rising, jumping 22% in the most recent week.
Still, the officials said, those are levels that are about one-third of where they were a year ago, largely thanks to immunity from vaccinations and prior infections.
But antibodies against Covid-19 wane over time, and many Americans are due for a tuneup. Only about 17% of those eligible got the bivalent vaccines last fall, the last time the immunizations were updated. Three vaccines are expected to be available this year. Two are mRNA vaccines, from Pfizer and Moderna, and a third protein subunit vaccine from Novavax. The Novavax vaccine uses an older type of technology that contains the spike protein of the virus that causes Covid-19, plus an ingredient that revs up the immune system to crank out antibodies against it.
Both vaccine technologies are well-understood and safe, and they have been proven in the real world and in clinical studies to reduce the risk of hospitalization and deaths from Covid-19, the officials said.
The FDA plans to grant full licensure for the Pfizer and Moderna vaccines for people 12 and older. Vaccines for children 11 and under, as well as the Novavax vaccine, will be available under an emergency use authorization, the officials said.
The vaccines have been updated to teach the body to fight the XBB.1.5 coronavirus subvariant. They are also expected to retain potency against closely related strains. All three vaccine manufacturers say testing shows that their vaccines are effective against EG.5, the currently dominant strain in the US.
While vaccines were previously provided for free by the government, this is the first time vaccines will be provided through the commercial market. Under the Affordable Care Act, most insurance plans are required to cover the full cost of vaccines, without co-pays.
People who don’t have insurance, or who don’t have enough insurance, can still get vaccines for free through a government bridge program.
“That bridge program will exist through a few channels,” CDC Director Dr. Mandy Cohen said in an interview with CNN last week. “Folks can go to a federally qualified health center or they can go to their public health department. … And then the third option is, we are working with pharmacy partners such as CVS, Walgreens, Walmart and others to have it available in the pharmacies as well.”
The details of the pharmacy program are still being worked out, and there may be a slight lag in getting free vaccines at some stores. But health departments and federally qualified health centers should have them right away, Cohen said.
People will only need to tell their vaccine provider that they don’t have insurance to qualify for the benefit, she said.
Cohen also said unless a person has never been vaccinated and never been infected, it’s probably better to wait until the new vaccines come out in September, rather than opting to get one of the older bivalent vaccines now. Getting a bivalent vaccine now might delay a person’s ability to get the new shot within the next few weeks, she cautioned. In a video Q&A posted online, she advised anyone in this circumstance to talk with their doctor or nurse practitioner about their individual risk.
In addition, there are still a significant number of Covid-19 tests in the Strategic National Stockpile. The government has been sending those free tests to nursing homes and assisted living facilities, federally qualified health centers, school, libraries and a number of other places that serve the public. That program will continue, officials said. There’s also the option to bring back Covidtest.gov, the website where people can order free tests.
The-CNN-Wire
™ & © 2023 Cable News Network, Inc., a Warner Bros. Discovery Company. All rights reserved.